Ineffective meibomian gland function can impact contact lens success.
A 33-year-old patient is in your chair for his annual eye examination and contact lens evaluation. Historically, he has responded that his lenses feel fine and has been a happy wearer. He now complains that the daily disposable lenses that he has been wearing for the past three years are bothering him. He says that he uses rewetting drops a few times each day and removes the lenses earlier in the evening to “give his eyes a break.” In fact, he finds himself frequently reaching for his glasses instead of wearing his contact lenses.
This scenario is not uncommon…but why does this previously successful wearer find his contact lenses dysfunctional? What steps should eyecare providers take to rectify the problem? Should practitioners reach for a different replacement modality? Should they switch lens brands or try a different material? Perhaps practitioners should re-examine the patient’s eyes and look at the ocular surface.
Over the past five years, we have managed this type of patient in a new way. Historically, practitioners would have reached for a new lens, blamed the contact lens solution, or blamed the patient for being noncompliant. We know that patients are habitual. If they don’t clean their lenses properly now, it will be hard to change that; and if they overwear their lenses, it will be hard to change that, too.
But the patient in this scenario reorders his lenses on an annual basis and comes into the office yearly. Most likely, his habits have not changed. However, his contact lens comfort has changed. He used to love wearing his contact lenses but can no longer wear them comfortably—the problem must therefore be the result of ocular surface changes.
TEAR FILM DISRUPTION
In our clinics, we find an ever-increasing number of patients able to utilize contact lenses for vision correction and disease management. Thanks to our industry partners, it’s a challenge to find a patient for whom contact lenses are not an option, because so many lens options are available including myopia management, single-vision lenses, astigmatism-correcting lenses, multifocals, and a plethora of specialty lens designs.
However, more than half of lens wearers report dry eye symptoms.1 Dryness and lens discomfort have been shown to be the leading causes of contact lens dropout,2 and a sharp decrease in successful contact lens wear occurs at approximately 45 years old.3 It is unfortunate that this age group sees such a sharp decline with functional lens wear, because they could greatly benefit from multifocal lenses as presbyopia increases their visual demands.
Despite evolving lens technology, novel materials, and improved multipurpose solutions, we continue to see high dropout rates. One aspect of lens wear that cannot be avoided is that the lenses alter the delicate balance of the tear film. We believe that an unstable, poor-quality tear film is the primary cause of lens discomfort.
We believe that the ocular surface should be able to support a contact lens and remain in homeostasis, despite the fact that the lens changes the composition of the tear film. The tear film is composed of mucins, proteins, and lipids, and it serves many purposes such as corneal lubrication, oxygenation, and functioning as the primary refractive component of the visual system. Introducing a contact lens to the ocular surface alters mucin and tear production4 and causes a thinned lipid layer on the anterior surface.5 The lipid layer helps protect the cornea from the shearing forces of the upper lid; when the lipid layer is deficient, the aqueous tear layer evaporates more quickly and, in contact lens wearers, creates a forward osmotic draw that dehydrates the contact lens6 and can cause symptoms of dryness.
RESTORING FUNCTIONAL LENS WEAR
Modality The first step in the treatment process is to ensure that patients are in a daily disposable modality. Daily disposable lenses have been shown to be the healthiest soft lens modality, with a lower incidence of corneal infiltrates7 and microbial keratitis8 as well as improved patient compliance compared to reusable lenses.9 Because daily disposable wearers use a fresh lens each day, they avoid lens protein accumulation that generally leads to increased cytokines, inflammation, and discomfort. Additionally, daily disposable lenses generally do not require the use of multipurpose solutions, making chemical agents responsible for cleaning, disinfecting, and conditioning the lenses a non-factor.
In some modalities, such as GP lenses, cleaning solutions cannot be avoided. For these patients, practitioners generally recommend a hydrogen peroxide cleaning solution to avoid potentially irritating preservatives. In short, the first step is to minimize the impact that the lenses have on the eyes. Once this is achieved, we investigate for additional causative factors.
Meibomian Gland Dysfunction (MGD) MGD also disrupts the quality of the ocular surface and can lead to unsuccessful contact lens wear. According to the International Workshop on Meibomian Gland Dysfunction, MGD is likely the leading cause of dry eye disease worldwide.10 In our contact lens patients complaining of dryness, MGD is always the first etiology that we rule out.
MGD is defined as “a chronic, diffuse abnormality of the meibomian glands, characterized by qualitative/quantitative changes in the glandular secretion.”10 MGD includes both hypersecretory and hyposecretory dysfunction, with the latter being most common.10
In some cases, such as in patients who have obvious obstructive MGD, a diagnosis can almost be made without evaluating those patients behind the slit lamp. These patients typically have inflamed lid margins and pouting, capped meibomian glands. Alternatively, we find that nonobvious MGD is much more common, but there are minimal-to-no signs of lid inflammation or capped meibomian glands, making the diagnosis of MGD difficult.
To best evaluate a patient’s glands for functionality, we utilize a meibomian gland expressor that delivers a steady pressure of 1.25g/mm2 to the external lid (Figure 1). This pressure exerts a force upon the meibomian glands that mimics that of a deliberate blink.11 In this way, we can assess whether the meibomian glands are functioning throughout a patient’s typical day.
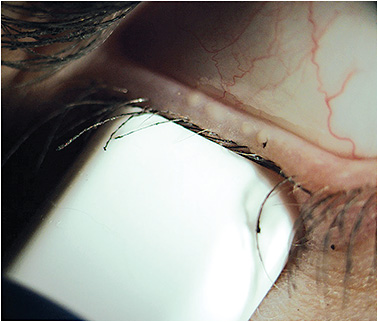
Figure 1. A calibrated pressure is being applied to the lower lid. Within the area of the meibomian gland evaluator, four glands are actively expressing oils.
Digital expression, expressor paddles, and cotton-tipped applicators can also be used to evaluate the quality of the meibum, but the force applied is not consistent or repeatable; instead, practitioners tend to apply increasing pressure to the lid until they see secretions, which elevates the perceived functionality.
We recently conducted a study in our office performing meibomian gland evaluation (MGE) on patients whom we had return to the clinic for a dry eye evaluation.12 Of 100 patients, the average MGE score was five functioning glands for each lid. Using the MGD diagnosis criteria of six or fewer glands, as outlined by the International Dry Eye WorkShop,13 78% of our study patients were diagnosed with MGD. Another study performed by Lemp et al found that 86% of dry eye patients had MGD as an underlying cause.14 Because of the high prevalence of MGD in dry eye patients and the fact that MGD can be present with nonobvious signs, it is vital to evaluate the gland function on every patient, but especially in those who are experiencing contact lens-related dryness.
Blinking Effective blinking also plays an important role as it relates to MGD and contact lenses. To understand this relationship, it is important to be familiar with the anatomy and physiology of the eyelid and meibomian gland. The meibomian gland lies along the internal margin of the lid. The pretarsal orbicularis muscle lies anterior to the gland, while the muscle of Riolan encapsulates the gland orifice. In an open-eye environment, Riolan’s muscle is contracted and blocks the orifice. It is thought that during a full and complete blink, the combined effect of Riolan’s muscle relaxing (opening the orifice) and the compression force of the orbicularis on the gland is responsible for meibomian gland expression.13
Contact lens wearers have been shown to have greater meibomian gland area loss15 than non-wearers do, abnormally thickened gland secretions, and lid margin anomalies.15,16 We believe that altered blinking is a significant contributor to these changes. Soft contact lens wearers tend to have more partial blinks than non-wearers do, especially during tasks for which concentration is necessary.17 We would expect full, effective blinks to be further reduced in corneal GP lens wearers compared to soft lens wearers and non-wearers. It is also well known that blink rate is significantly reduced while we use computer screens, so by coupling the growing number of hours that we are using digital devices and the number of patients opting to be fit into contact lenses, this may be setting the stage for a higher incidence of MGD.
SIT FOR SUCCESS: SCREEN, IDENTIFY, TREAT
Let’s say that in our 33-year-old patient complaining of contact lens discomfort, we identify that he has MGD based on a MGE score of 5 OD and OS. We’ve established that he is wearing the lenses as instructed and that we find no additional causative factors of dry eye.
There is no shortage of treatment options available for our dry eye patients; in fact, because there are so many options, it is often difficult to know where to start. In our clinics, we believe in a targeted approach. The patient has MGD, so our goal must be to restore function to those glands, thereby improving the quality of the ocular surface and tear film, allowing for the eye to once again support a contact lens.
Whichever treatment approach practitioners choose to utilize in their clinics, it is imperative to monitor meibomian gland function. Many treatments have improved dry eye questionnaire responses, tear breakup times, and even lipid layer thickness measurements, but unless the meibomian glands are flowing again, the patients still have MGD. Therefore, it necessary to emphasize this as part of a treatment plan.
In one 2018 study, contact lens wearers who had MGD and symptomatic dry eye were evaluated before and after a single treatment of vectored thermal pulsation.18 Three months after treatment, the mean number of functioning meibomian glands increased by more than three times from baseline, dry eye symptom scores decreased by 58%, and comfortable contact lens wear time more than doubled.18
In our clinic, after treating the MGD, we emphasize the importance of regimented at-home treatment to maintain the improved results. We educate the patients on the importance of blinking and instruct them to perform functional blinking exercises, have them utilize a heat mask daily to help maintain meibomian gland function, and have them add a daily lid scrub to maintain lid and lash hygiene.
THE BOTTOM LINE
Many contact lens wearers wish to continue wearing their lenses for the long term. And, from a practice management standpoint, it benefits eyecare practitioners to ensure that this is possible.
To provide the opportunity for functional lens wear for patients and to provide optimal eye care, we screen all patients with dry eye questionnaires and vital dye staining. After evaluation, if we find that patients exhibit signs of dry eye, we will have them return for a more detailed dry eye evaluation to identify additional causative factors that staining and meibomian gland expression may not precisely show. By adding an additional minute to each exam, you can identify dry eye and meibomian gland dysfunction in its earliest stages, initiate treatment, and allow patients years of comfortable lens wear. It is very simple: functional meibomian glands lead to functional contact lenses. CLS
READ more of my article here at Contact Lens Spectrum





